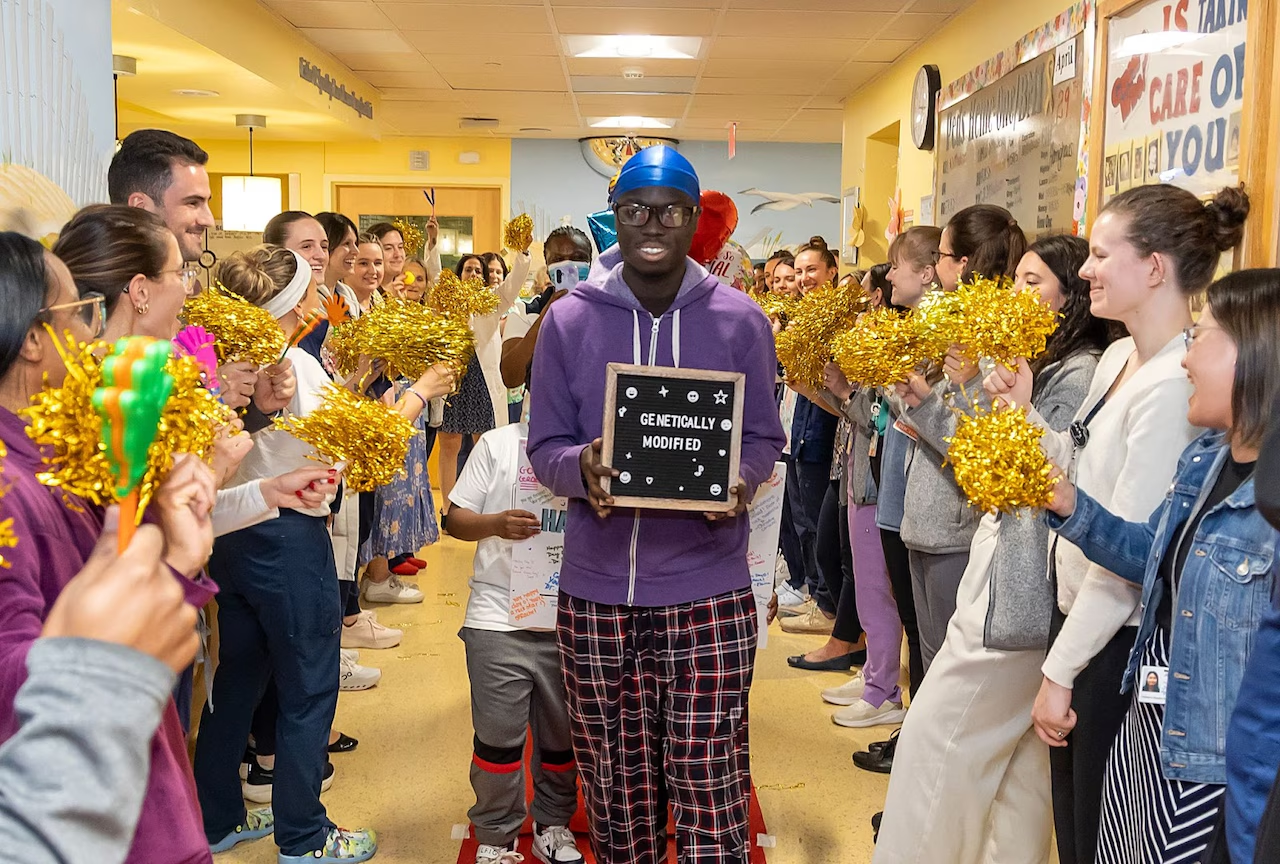A teenager walked out of Hackensack Meridian Health Hackensack University Medical Center, (HUMC), the first patient successfully treated with Lyfgenia, a newly approved gene therapy for sickle cell disease (SCD).
Marking a milestone in curative care, Gerald Quartey, 18, was treated with Lyfgenia at Joseph M. Sanzari Children’s Hospital, located at HUMC in Hackensack, New Jersey.

He is the first patient at HMH HUMC to complete the federally approved Lyfgenia therapy since its FDA authorization in December 2023, the hospital said in a statement last week.
An inherited blood disorder, Gerald’s mother Evelyn Quartey carries the sickle cell trait, as did her ex-husband and oldest son, Emmanuel, who died of the disease 16 years ago when he was 7. One in 13 African Americans carry the sickle cell trait and 1 in 365 are born with the disease—more than any other ethnic group in the U.S.
SCD By The Numbers
Lyfgenia, formerly known as LentiGlobin, is among the most expensive remedies in U.S. history, with a list price of $3.1 million for the one-time treatment, plus the additional costs of other required procedures, including chemotherapy before the treatment. The FDA approved it in December 2023 for patients aged 12 and older.
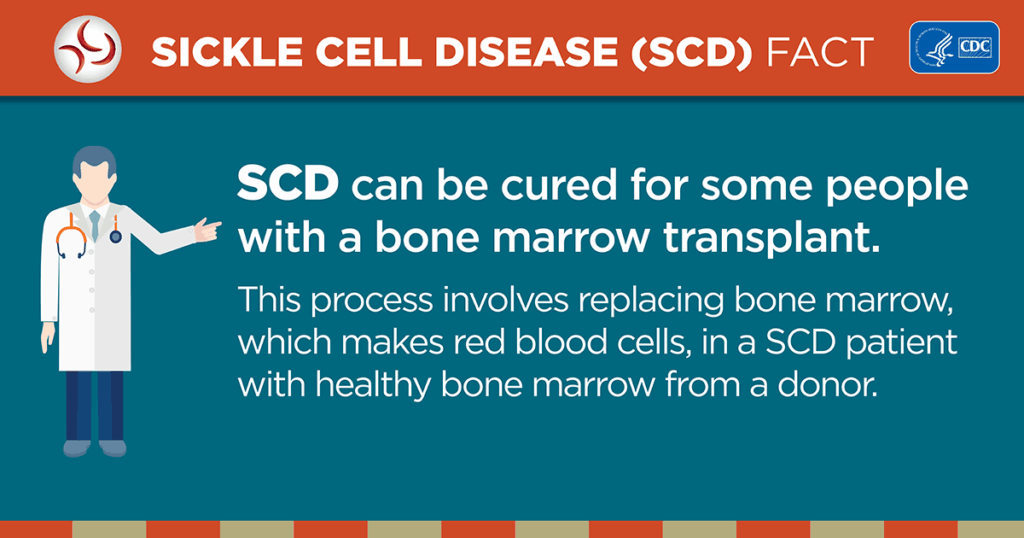
This is not just a physical disease — it impacts every part of a patient’s life and family,” said Dr. Stacey Rifkin-Zenenberg, DO, FAAP, FAAHPM, a pediatric hematologist/oncologist and a lead investigator in clinical trials for curative sickle cell treatments.
“To now be able to offer a real cure is extraordinary,” Rifkin-Zenenberg added.
New Jersey has about 5,000 pediatric and adult SCD cases combined, ranking in the top 10 states nationwide, Rifkin-Zenenberg said.
Transformative Treatment Journey
After repeated complications from sickle cell disease, Gerald Quartey’s road to recovery began last year with a referral for advanced care at Joseph M. Sanzari Children’s Hospital. There, Gerald’s medical care team collected his stem cells for genetic modification to correct the defect responsible for sickle-shaped red blood cells.
He returned to the hospital for a month-long stay in the spring, receiving chemotherapy to prepare his bone marrow before the re-engineered cells were infused back into his body.
“This is a transformational moment in the treatment of sickle cell disease,” said Dr. Lisa Tank, M.D., FACP, CMD, Chief Medical Officer at Hackensack University Medical Center
“It speaks to our commitment to bringing cutting-edge therapies to the bedside and delivering patient-centered care backed by scientific rigor,” Dr. Tank added.
Joint Partnership Within HUMC
The gene therapy was delivered through the joint program offered by Joseph M. Sanzari Children’s Hospital and the John Theurer Cancer Center, both designated Qualified Treatment Centers for Lyfgenia by Bluebird Bio. The FDA gave approval of Lyfgenia to Bluebird Bio Inc.
Hackensack was the first site in New Jersey approved to administer the treatment.
The therapy is the second pediatric gene therapy completed at HUMC this year. Earlier, the team treated a young girl with beta thalassemia using Zynteglo, another one-time gene therapy approved alongside Lyfgenia.
“This campus has a decades-long legacy in curing blood disorders,” said Mark Sparta, PT, MPA, FACHE, president of Hackensack Meridian Health’s Northern Market.
“From bone marrow transplants to gene therapy, we’ve led the way in innovation and compassionate care for patients living with sickle cell,” Sparta added.
SCD: An Inherited Blood Disorder
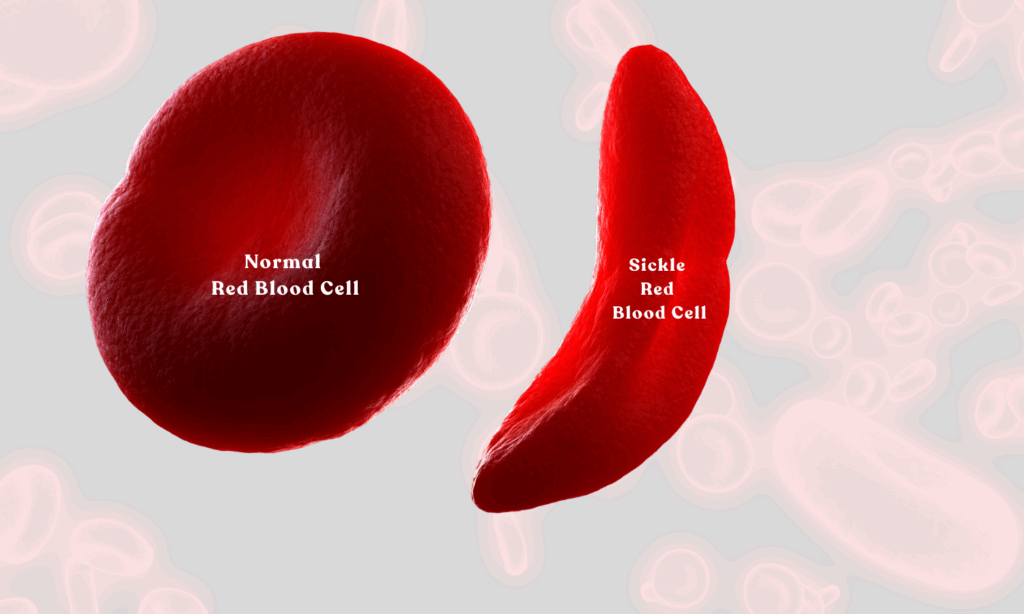
Sickle Cell Disease is an inherited blood disorder that alters the shape of red blood cells, forcing them into a rigid, sickle-like form. The abnormal shape limits their ability to carry oxygen and can cause the cells to clump together, blocking blood flow and triggering intense pain, organ complications, and a higher risk of infection, according to the U.S. Centers for Disease Control and Prevention.
The CDC data shows that SCD affects one in 2,070 newborns overall and one in 350 non-Hispanic Black newborns.
The state Department of Health reports the disease primarily affects African American communities. Sickle cell disease is an inherited disorder where abnormally shaped red blood cells block blood flow, leading to pain, anemia, organ damage, and other serious complications. Lyfgenia modifies a patient’s own stem cells to produce normal hemoglobin, reducing the number and severity of vaso-occlusive events (VOEs), also called sickle cell crises.
SCD occurs in about 1 out of every 365 Black or African American births and about 1 out of every 16,300 Hispanic American births. About 1 in 13 Black or African American babies is born with sickle cell trait (SCT, inheritance of a sickle cell gene from only one parent), according to the Center for Disease Control.
“Sickle cell disease is a rare, debilitating and life-threatening blood disorder with significant unmet need, and we are excited to advance the field especially for individuals whose lives have been severely disrupted by the disease by approving two cell-based gene therapies today,” said Nicole Verdun, M.D., director of the Office of Therapeutic Products within the FDA’s Center for Biologics Evaluation and Research.
“Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited,” Verdun said.
Last year, NJ Gov. Phil Murphy established a $10,200,000, three-year sickle cell disease pilot program under the NJ Department of Health (DOH), in consultation with the Department of Human Services.
The DOH selected Federally Qualified Health Centers for participation in the pilot program on a competitive basis using criteria established by the Commissioner of Health, the state said last year.
State’s Oldest & Largest Program
Hackensack Meridian Children’s Health operates the oldest and largest pediatric sickle cell cure program in New Jersey, in partnership with the John Theurer Cancer Center.
The joint program is accredited by the Foundation for the Accreditation of Cellular Therapy (FACT) and has cured more than 80 patients over the past two decades through stem cell and now gene therapies.

In 2019, Joseph M. Sanzari Children’s Hospital and the John Theurer Cancer Center, on the Hackensack University Medical Center campus, became one of only a few U.S. hospitals, and the only in New Jersey to offer a national LentiGlobin gene therapy clinical trial. At an estimated $3.1 million, Lyfgenia is one of the highest prices ever for the treatment of a disease.
Hackensack Meridian Children’s Health is the first hospital in New Jersey, and in the New York metropolitan area, to administer the Bluebird-manufactured Lyfgenia™ treatment as a curative gene therapy treatment for sickle cell disease (SAD).
FDA Approval
Lyfgenia and Casgevy are the first cell-based gene therapies for the treatment of sickle cell disease (SCD) in patients 12 years and older. These treatments are the first gene therapies approved for SCD in the U.S.
Additionally, Casgevy is the first FDA-approved treatment to utilize a type of novel genome editing technology, signaling an innovative advancement in the field of gene therapy, the FDA said in a statement.
Both the Casgevy and Lyfgenia applications received Priority Review, Orphan Drug, Fast Track and Regenerative Medicine Advanced Therapy designations.
The treatment is made from the “patients’ own blood stem cells, which are modified and given back to the patient as a one-time, single-dose infusion as part of a hematopoietic stem cell transplant,” according to the American Society of Gene + Cell Therapy.
Sickel Cell Disease
Sickle Cell Disease is the largest inherited disease in the United States, affecting 1 out of every 865 African Americans and 1 out of every 16,300 Hispanic-American births. New Jersey has an estimated 2000 cases of SCD, with 80-90 births occurring annually, according to Tackle Kids Cancer at HUMC.
Sickle Cell Disease (SCD) is the largest inherited disease in the United States, affecting 1 out of every 865 African Americans and 1 out of every 16,300 Hispanic-American births. New Jersey has an estimated 2000 cases of SCD, with 80-90 births occurring annually.